Fall/Winter 2024

AMP Files Lawsuit Against FDA to challenge Final Rule
On April 29, 2024 the U.S. Food and Drug Administration finalized a rule "Medical Devices; Laboratory Developed Tests" (Docket No. FDA-2023-N-2177), that seeks to regulate all laboratory developed testing procedures (LDPs) as medical devices. In response to the final rule that would upend the laboratory community across the country and has devastating consequences for patient access to care, AMP has filed a lawsuit against the FDA.
THE LAWSUIT DOES NOT DELAY THE IMPLEMENTATION OF THE FINAL RULE. AMP member and pathologist, Dr. Michael Laposata, is a co-plaintiff in this case which will be heard in the Eastern District Court of Texas. AMP’s main arguments are that FDA does not have the statutory authority to regulate LDPs and by finalizing the rule, the FDA is encroaching on the practice of medicine. Please find the tentative briefing schedule below
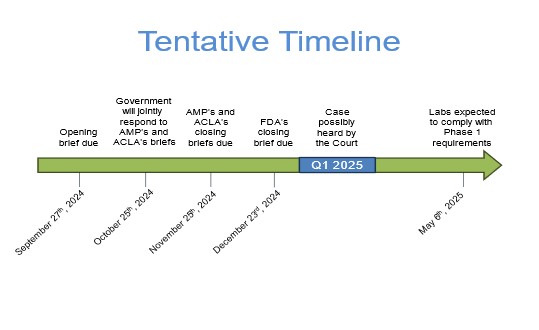
AMP is hopeful that the case will be heard in early 2025 in Dallas, TX. We do not yet know approximate dates for when a decision may be reached by court.
HOW YOU CAN HELP?
The best way you can support AMP in its efforts to advocate on your behalf in response to the FDA’s Final Rule is first and foremost to renew your membership or to join as a new member. AMP’s strength lies in its membership and the expertise, innovation, and advocacy that our members bring to the field. Alternatively, AMP is a 501(c)(3) and can accept donations from companies and individuals. AMP is accepting unrestricted donations in support of our public policy and advocacy projects. We recommend donating to the Strategic Initiatives Fund which serves to support AMP’s greatest areas of need: amp.org/donate
AMP Appropriations Report Language included in House Bill
AMP is thrilled to share that our appropriations report language was included in the U.S. House Appropriations Committee on Agriculture, Rural Development, and Food and Drug Administration Fiscal Year 2025 bill. The language (below) which would halt the implementation of the final rule, can also be found on page 84 . While report language does not become law, it is extremely powerful and sends a very strong message that the House Appropriations Committee opposes FDA's rule. The Senate Appropriations Committee did not include AMP’s language in their report. However, one chamber can defer to another depending on policy issue Please note: the spending bills will most likely be considered at the end of the year, therefore THE IMPLEMENTATION OF THE FINAL RULE IS NOT DELAYED. Please prepare your lab accordingly for the new FDA regulations. AMP strongly recommends you seek licensed counsel and/or regulatory affairs specialists to assist you with the various compliance stages of the Final Rule.
Report Language:
Laboratory Developed Tests.-The FDA's final rule on Laboratory Developed Tests (LDTs) puts forth a proposed regulatory framework that is a significant shift in the way LDTs are regulated and changes expectations for patients, doctors, and laboratories for the first time since the Clinical Laboratory Improvement Amendments Act was passed in 1988 at the risk of greatly altering the United States' laboratory testing infrastructure and reducing patient access to information that informs their healthcare decision making. The Committee directs the FDA to suspend its efforts to implement the rule and continue working with Congress to modernize the regulatory approach for LDTs.
AMP 2023 CLIA Modernization Proposal
AMP’s CLIA Modernization Proposal maintains that CMS has the authority to oversee LDP regulation through the Clinical Laboratory Improvement Amendments (CLIA) of 1988. The proposal would definitively remove FDA oversight of LDPs and expand several aspects of the current regulations to reflect the modern field of laboratory medicine. This proposal will preserve CMS oversight of LDPs in the most streamlined, cost-effective way that will not disrupt patient access to testing. AMP converted the proposal into legislative text and you can view our Frequently Asked Questions (FAQs) here and our Section-by-Section analysis here. AMP continues to collaborate with with stakeholders and lawmakers to move this initiative forward.
Image source: https://bit.ly/3mSMInk
Patent Eligibility Restoration Act (PERA) seeks to patent genes, overturn AMP v. Myriad
Patent Eligibility Restoration Act (PERA)
The Patent Eligibility Restoration Act, 2023 was introduced by Senator Coons (D-DE) and Tillis (R-NC) last year. This bill would overturn the AMP v. Myriad Supreme Court case which protects genetic sequences from becoming patent-eligible. On September 19, 2024, the Senate Judiciary Committee reviewed PERA in the Executive Committee Business session and a Committee-level vote is expected next week which would determine if the bill moves forward or not. U.S. Representatives Scott Peters (D-CA) and Kevin Kiley (R-CA) recently introduced the same version of the bill in the House on September 6, 2024. AMP continues to engage key Congressional offices in efforts to prevent PERA from moving forward.

AMP Annual Molecular Pathology Economics Summit
AMP hosted the fourth annual Economics Summit on August 16th, 2024 which seeks to identify barriers to appropriate reimbursement for molecular pathology procedures; examine the impact of these barriers on various stakeholders and patient access to care; and propose potential solutions and/or novel approaches to overcoming barriers to implement shared policy goals from participating stakeholders in oncology, infectious diseases, and inherited conditions. Attendees included stakeholders from clinical laboratories, pharmaceutical industry, diagnostic manufacturers and patient advocate communities to discuss coding/pricing and coverage challenges and provide potential solutions and action items. A Summary of the event will be released shortly.
Recent Comment Letters:
September 18, 2024 - Updated Stakeholder Letter Opposing S. 2140, Patent Eligibility Restoration Act (PERA) 2023
September 13, 2024 - AMP-CAP Comments on draft Local Coverage Determination DL39919 MolDX: Non-Next Generation Sequencing Tests for the Diagnosis of BCR-ABL Negative Myeloproliferative Neoplasms.
July 30, 2024 - AMP response to Cures 3.0 RFI
July 3, 2024 - AMP Comments on FDA Enforcement Discretion on IVDs During a 564 Emergency
July 3, 2024 - AMP Comments on FDA Enforcement Discretion on IVDs in Absence of a 564 Emergency
July 1, 2024 - AMP comments on Clinical Laboratory Fee Schedule Preliminary Gapfill Determination
April 4, 2024 - AMP Response to Sen. Cassidy RFI on Regulation of Clinical Tests
March 13, 2024 - Stakeholder Letter Opposing the Patent Eligibility Restoration Act of 2023 (PERA)
March 1, 2024 - AMP-CAP comments on Molecular Assays for the Diagnosis of Cutaneous Melanoma
January 22, 2024 - AMP Submitted Testimony for Senate Hearing Regarding The Patent Eligibility Restoration Act of 2023 (PERA)
The AMP Economic Affairs Committee and Professional Relations Committee work diligently to provide input to Congress and relevant agencies on all issues affecting regulation and reimbursement of molecular procedures. You can peruse all recent comment letters here.