Reports & White Papers
-

- Molecular Pathology Economics 101
-
August 2020 | AMP convened a multidisciplinary working group to describe the complex landscape of molecular pathology economics and highlight opportunities for member engagement.
-
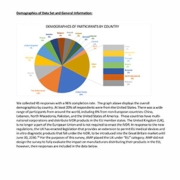
- Impacts of the European Union In Vitro Diagnostic Regulation Survey
-
February 2024 | On May 5, 2017, the European Union (EU) published two regulations that require medical device and in vitro diagnostic manufacturers that distribute products in the EU to adhere to new standards. AMP membership sought to understand the global scope and impact this would have on clinical laboratories and patient access to care.
-

- PAMA Impact Survey
-
March 2023 | AMP sought support in understanding the impact of PAMA on laboratory testing through a quantitative survey deployed to its membership. ClearView worked with AMP to develop a multi-step approach to a quantitative survey and analysis to answer the key questions and accomplish AMP’s objectives. Read the results.
-

- Economics of Testing During a Public Health Emergency: Lessons learned from two years of COVID-19
-
March 2022 | In this white paper, AMP Economic Affairs Committee subject matter experts reflect on the first two years of the COVID-19 pandemic and the unique economic challenges faced by laboratories, particularly at the onset. Policies on coding, coverage, and pricing enacted to respond to the COVID-19 pandemic for SARS-CoV-2 molecular diagnostic tests are examined, and recommendations for how the challenges laboratories faced can be prevented (or at least mitigated) in the future are provided.
-

- Analysis of Effort in Molecular Test Interpretation
-
March 2021 | The evolving molecular test landscape is driving more demand for not only tests but complex interpretation and reporting services. There are different fee schedules for lab tests and physician services, respectively, with minimal values assigned to interpretation. ClearView worked with AMP to examine the burden of molecular test data analysis, interpretation and reporting and its impact on lab services. Qualitative and quantitative assessments were conducted to characterize data interpretation / reporting burdens and associated barriers to testing. See the results of the quantitative survey
-

- SARS-CoV-2 Testing Survey Results
-
February 2021 | Access the results of three surveys:
-
Cancer Diagnostic Testing Amidst COVID-19 Pandemic Survey
-
AMP August 2020 SARS-CoV-2 Diagnostic Testing Survey
-
AMP April 2020 SARS-CoV-2 Diagnostic Testing Survey
Links to recordings of relevant webinars also are available.
-